News Release
-
May 10, 2022
Notice of the URL Change
Old URL: http://www.zenoaq.jp/zenogen_pharma/en/
New URL: https://www.zenogenpharma.com/en/ - April 01, 2022 ZENOGEN PHARMA Executes Marketing Collaboration Agreement with Iwai North America Inc.
- July 1, 2021 Notice of Company Name Change and Appointment of Officers
-
February 16, 2021
ZENOAQ Group to Change to Holding Company Structure
Establishment of ZENOAQ HOLDINGS CO., LTD.
[Click here for details] - June 26, 2018 Our website has just opened.
Product Information
- January 30, 2026 CELLBANKER's References page has been updated.
- October 30, 2025 CELLBANKER's References page has been updated.
- July 24, 2025 CELLBANKER's References page has been updated.
- April 30, 2025 CELLBANKER's References page has been updated.
- January 30, 2025 CELLBANKER's References page has been updated.
- November 12, 2024 CELLBANKER's References page has been updated.
- July 31, 2024 CELLBANKER's References page has been updated.
Overview
As a part of this universe, we live our daily lives while being exposed to external changes every day. While technology has allowed our lives to become more and more convenient, problems that we must respond to societally, such as declining birthrate, aging population, and global environmental issues, have continued to grow. In a time such as now, when the globalism of socio-economics has become the norm, we must be physically and mentally prepared to adapt to a wide variety of changes. Within this background, ZENOGEN aims to be a corporation that supports medical care from the pharmaceutical perspective. By applying science to people’s lives, we hope to contribute to a society where people can live even just a little longer, as well as lead full and healthy lives. Medicine, which begins with pharmaceuticals, is advancing every day. Meanwhile, the value of medicine is changing with the times, and we will continue to contribute to society by understanding those changes and creating new value in medicine that leads to the medical care of the future. We will establish technology that leads to future pharmaceuticals that are effective against difficult-to-treat diseases such as cancer and intractable diseases, and we will work together with like-minded partners to develop cures. Furthermore, we are committed to generating value for better pharmaceuticals by providing comprehensive support through our products and services.
Business information
-
Antibody therapeutics are not only effective for patients who experience insufficient effects from conventional drugs, but can also reduce the risk of side effects via bonding to specific targets. Antibody therapeutics for cancer and the field of intractable disease are being continuously developed and used for treatment at clinical sites all around the world.
In recent years, many kinds of antibody therapeutics have been increasing in number due to innovations in antibody engineering technology. The strengths and weaknesses of antibodies have a significant effect on the results of the ensuing pharmaceutical development.
By establishing a technological platform that can effectively produce specifically targeted and functional antibodies, and by expanding it to the development of new drugs as well as other products and services, our company contributes to the creation of better pharmaceuticals and diagnostic drugs.
Antibody
-
Antibody therapeutics are not only effective for patients who experience insufficient effects from conventional drugs, but can also reduce the risk of side effects via bonding to specific targets. Antibody therapeutics for cancer and the field of intractable disease are being continuously developed and used for treatment at clinical sites all around the world.
In recent years, many kinds of antibody therapeutics have been increasing in number due to innovations in antibody engineering technology. The strengths and weaknesses of antibodies have a significant effect on the results of the ensuing pharmaceutical development.
By establishing a technological platform that can effectively produce specifically targeted and functional antibodies, and by expanding it to the development of new drugs as well as other products and services, our company contributes to the creation of better pharmaceuticals and diagnostic drugs.
Antibody
-
The history of vaccines dates back to the end of the 18th century with Edward Jenner’s discovery of the smallpox vaccine, and continues to this day, as various vaccines have contributed to medical care by preventing infectious disease.
In recent years, the complicated workings of the body’s immune system have become clearer. Due to many illnesses occurring as a result of breakdown of the immune system, vaccines are extensively used in medical care.
Our company has developed a particulate polymer that is able to greatly increase activation of the immune system. This polymer has the ability to successfully increase humoral and cellular immunity, and we have found that it has the potential to perform and be safely used to excellent effect. We look to propose new treatment concepts by constructing a highly functional adjuvant based on this polymer, developing safe and highly effective vaccines, and expanding its use to other products and services.
Adjuvant
-
CELLBANKER is a series of cell cryopreservation media and has been our leading product since it became commercially available in 1991. The medium achieves over 80% cell viability after thawing for most cell lines, and occupies over 70% of the share in experimental reagents in Japan. At present, we are developing novel applications of the CELLBANKER series to meet the needs of current research trends. In particular, the discovery of induced pluripotent stem cells (iPS cells) and related research efforts in recent years are leading the field of regenerative medicine as a novel industry to realize practical applications.
Regenerative medicine has opened new avenues for curing patients with difficult-to-treat diseases and diseases that are resistant to conventional therapies.
We are transforming the CELLBANKER series from being experimental in nature to being applicable in regenerative medicine in terms of quality and functionality, as well as addressing current issues with cryopreservation in basic research and clinical settings.
Cellbanker
-
Contract Manufacturing of Biologics
We are collaborating with ZENOAQ, our group company, to provide services for contract manufacturing of human biopharmaceuticals at ZENOAQ biological manufacturing plant permitted by the Pharmaceuticals and Medical Devices Agency (PMDA, Japan). The facilities also comply with cGMP and PIC/S GMP guidelines. We are able to carry out contract manufacturing on a scale that meets the needs of the client, ranging from seed cultures to mass quantity cultures of up to two thousand liters. Having the manufacturing plants to handle production of various products, we also work flexibly according to our clients' requests and goals, from conducting a wide variety of tests, including raw material tests, in-process tests, and product tests, to cell bank production and GMP manufacturing.
We provide support for these contract services from inquiries to contract conclusion and provision of deliverables.
Biomedicine
CELLBANKER series
STEM-CELLBANKER
GMP grade
- 100ml
- 20ml
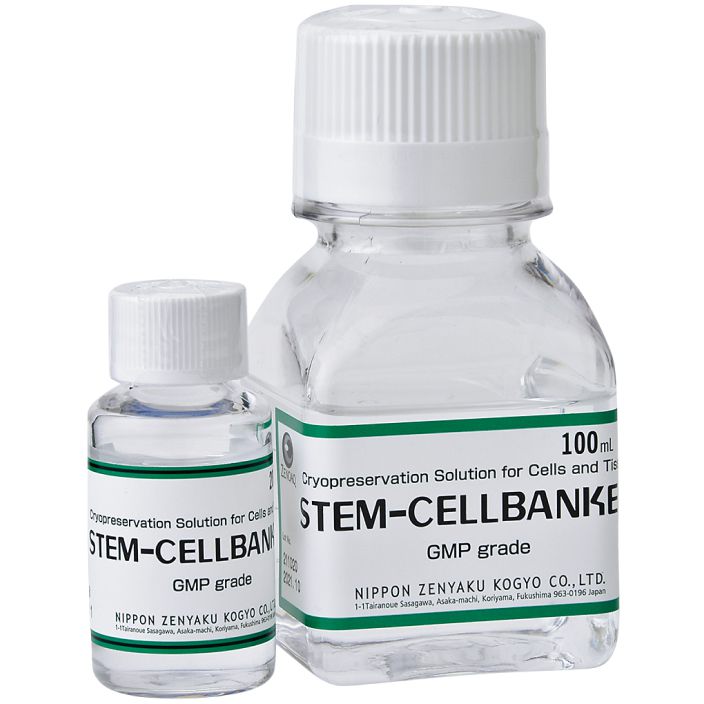
- Reference
- Serum-free
- [ Feature ]
- Manufacturing and quality control processes for this product are performed under GMP conditions. STEM-CELLBANKER GMP grade is a cryopreservation medium for cells and tissues, and is optimized for storage of valuable cells such as ES cells, iPS cells, and mesenchymal stem cells.
STEM-CELLBANKER
DMSO Free GMP grade
- 100ml
- 20ml
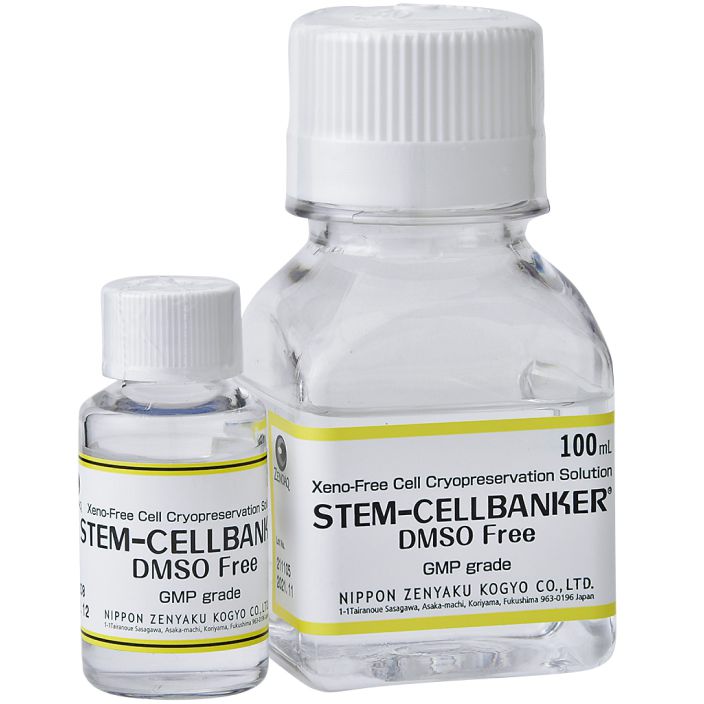
- Reference
- Serum-free
- [ Feature ]
- STEM-CELLBANKER DMSO Free GMP grade is a chemically defined (all components are known and chemically defined) and optimized cell cryopreservation medium. Its serum meets the criteria for being xeno-free, meaning it does not contain any animal-derived components. Its components meet the official specifications of the Japanese, United States, and European Pharmacopeia.
NEWSTEM-CELLBANKER
EX GMP grade
- 100ml
- Reference
- Serum-free
- [ Feature ]
- STEM-CELLBANKER® EX GMP grade (SCB EX) is a DMSO-type cryopreservation solution that does not contain serum or animal-derived components (Xeno-Free) and uses proven ingredients for intravenous injection. SCB EX can preserve human adipocyte-derived stromal/stem cells and human hematopoietic stem cells. The composition of SCB EX is the same as HSC-BANKER® GMP grade.
HSC-BANKER
GMP grade
- 15ml
- Reference
- Glass vial
Serum-free
- [ Feature ]
- HSC-BANKER GMP grade is a ready-to-use cryopreservation medium for hematopoietic stem cells. It is entirely made in Japan, and its manufacturing and quality control processes are performed under GMP conditions. To ensure that it is sterility, all manufacturing processes in which the product is exposed to the environment are conducted in a Grade A environment. It is suited for storage of stem cells derived from the umbilical cord.
CELLBANKER 1
- 100ml
- 20ml
- Reference
- Serum contained
- [ Feature ]
- CELLBANKER 1 enables rapid freezing in a deep freezer without adjusting reagents, thus saving time and effort. It achieves high cell viability after thawing.
CELLBANKER 2
- 100ml
- 20ml
- Reference
- Serum-free
- [ Feature ]
- CELLBANKER 2 is a serum-free cell cryopreservation medium. Its efficacy is equivalent to media that contain serum. Since it does not contain proteins such as serum and albumin, there is no contamination or effect on cells from these proteins.
CELLOTION
- 100ml
- Reference
- Cell washing and recovery
- [ Feature ]
- CELLOTION is a chemically defined (all components are known and chemically defined) cell washing and recovery solution. By reducing the loss of cells after washing, it achieves a high cell recovery yield. It is free of serum, protein, and sugars, and is thus suitable for washing cells, tissues, and organs.
Corporate Profile
| Corporate Name: | ZENOGEN PHARMA CO., LTD. |
|---|---|
| Office: | 1-1 Tairanoue, Sasagawa, Asaka-machi, Koriyama, Fukushima, 963-0196 Japan |
| Representative: | Masato Fukui |
| Date of Establishment: | April 1985 |
| Capital: | 100 million yen |
| Core Business: | Research, development, manufacturing, and sales of products and services relating to life science field. |
| Group Company: | ZENOAQ HOLDINGS CO., LTD. NIPPON ZENYAKU KOGYO CO., LTD. (ZENOAQ) |